Background: Different aspects of the gut microbiota (GM), including diversity, abundance, and functionality, have been linked to the development, progression, response, and adverse events to therapy in plasma cell neoplasms (PCN). The primary goal of this study was to further investigate the relationship between the composition of the GM, focusing on the genus Faecalibacterium that contains the most common anaerobic commensal bacterium in the GM ( Faecalibacterium prausnitzii), and clinical outcomes of patients with PCN undergoing autologous hematopoietic stem cell transplant (HSCT).
Methods: GM samples were collected prospectively between 2017 and 2021 at our institution as part of a phase III, randomized clinical trial including patients with malignant hematologic diseases undergoing either chemotherapy or transplant. Fecal samples were obtained at pre (baseline), mid (defined as at 1 week from baseline), and end (defined as at engraftment, absolute neutrophil count > 500 cells/mm 3) autologous HSCT and processed by 16S rDNA sequencing (V1-V3) using Illumina MiSeq to determine Faecalibacterium abundance at each prespecified time point. Progression free survival (PFS) and overall survival (OS) were computed via Kaplan-Meier estimate.
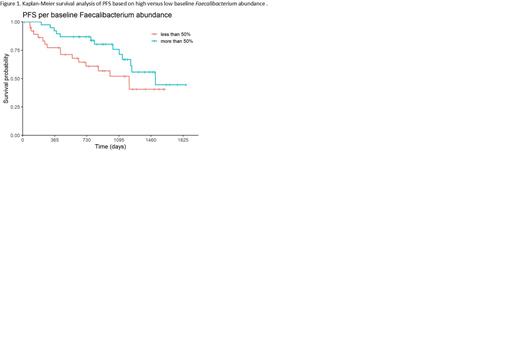
Results: 83 evaluable patients underwent autologous HSCT for PCN, 54 (65%) as part of frontline therapy. 79 (95%) of the transplant recipients carried a diagnosis of Multiple Myeloma. Melphalan was used as the conditioning regimen, with 54 (65%) of the patients receiving a dose of 200 mg/m 2 (versus 140 mg/m 2). The median age of the patients was 64 (range, 31-79) years and 50 (60%) were men. With median time to follow up for survivors (n= 82, alive > 1 month post HSCT) of 32 (range, 0.7-61) months, the median PFS was 40 months and median OS was not reached. Faecalibacterium relative abundance was 3.5%, 1.7%, and 2% of the GM at pre, mid, and end transplant, respectively. Higher baseline Faecalibacterium abundance was significantly associated with superior PFS (n= 79) (HR= 0.92, p= 0.01) and OS (HR= 0.88, p= 0.008). Patients were partitioned into 2 groups, those with pre-transplant Faecalibacterium abundance above median and below median, to generate representative Kaplan-Meier curves for PFS (Figure 1). Greater reduction in Faecalibacterium abundance during transplant, and especially from pre to mid transplant, were significantly associated with worse PFS (n= 75) (HR= 1.08, p= 0.007; HR= 1.10, p= 0.004, respectively) and OS (HR= 1.09, p= 0.021; HR= 1.09, p= 0.04, respectively). There was no significant relationship between Faecalibacterium abundance and PFS at mid (n= 83) (HR= 1, p= 1), end (n= 83) (HR= 1.01, p= 0.7), or notably mid to end transplant (n= 75) (HR= 1, p= 0.9). Levels of the bacteria also did not associate with OS from mid to end transplant (n= 75) (HR 1.01, p= 0.8).
Conclusions: Our study indicated that Faecalibacterium abundance in the GM changed rapidly early after autologous HSCT in patients with PCN likely due to melphalan administration. Higher baseline levels and smaller reduction in Faecalibacterium abundance predicted superior PFS. Further follow up with more mature data is needed to confirm OS associations. These findings support the need for further investigation into the role of GM and possible interventions to modify GM to improve outcomes to autologous transplant in patients with PCN.
Disclosures
Dean:Moffitt Cancer Center: Patents & Royalties: unlicensed patents (no royalties) in the area of cellular immunotherapy. Wingard:F2G: Consultancy; Celgene: Consultancy; Orca: Consultancy; Takeda: Consultancy; Cidara: Consultancy.